Isotopes: what to remember

The isotope is an atom whose properties give it a strategic place in many fields, such as health, industry and fundamental research. Let’s discover these strange "materials" at the heart of technological innovation...
What is an isotope?
The matter that surrounds us, water, air, or even living beings, is made up of atoms, particles invisible to the naked eye. Atoms are composed of a nucleus, around which electrons orbit.
Inside the atom's nucleus are particles called neutrons, which are bound to protons. The cumulative number of protons and neutrons (together known as nucleons) in an atomic nucleus is the atomic mass of the atom.
When we talk about an isotope,
we distinguish a type of atom that has the same number of protons but a different number of neutrons.
The properties of isotopes
Inside the atomic nucleus, the number of protons defines the chemical properties of the atom. Two atoms with the same number of protons are called isotopes. They belong to the same chemical element because they have the same
number of protons in the nucleus.
However, isotopes, which belong to the same chemical element, are distinguished by different physical properties because of the number of neutrons they have. The neutron is an elementary
particle of neutral electric charge, which can be stable or unstable.
Each isotope of the same chemical element thus shares the same atomic number (Z), which corresponds to the number of protons they have.
However, they are distinguished
from each other by their atomic mass number (neutrons + protons); the number of neutrons they have is different.
An example - isotopes of carbon
Take the example of the isotopes of carbon: carbon-12 and carbon-14. Both are isotopes of the same chemical element, carbon. These isotopes both have 6 protons. However, the number of neutrons they have is differentCarbon-12 has 6 neutrons and carbon-14 has 8 neutrons. Because of the number of neutron they have, they have different physical properties.
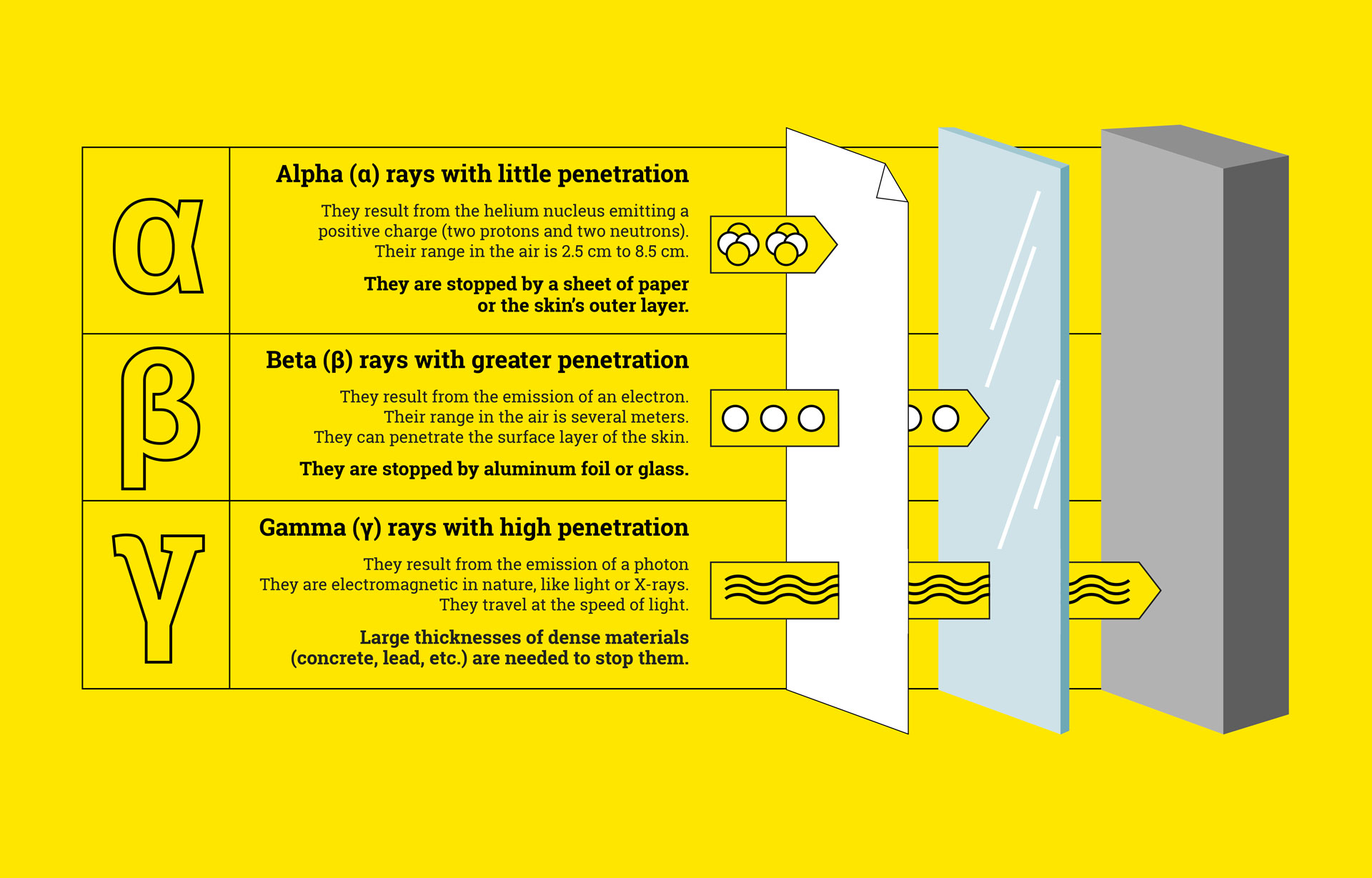
Carbon-14 is considered an unstable isotope. It decays and emits radioactivity in the form of beta rays. It has a total of 14 nucleons (6 protons and 8 neutrons).
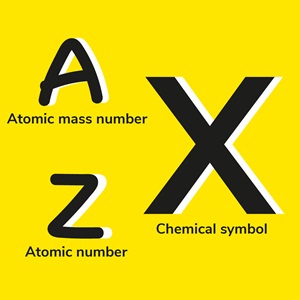
In the notation of carbon 14, we therefore write 14
6
C, C being the chemical symbol of carbon, 14 its atomic mass number (protons + neutrons) and 6 corresponds to its atomic number,
i.e. its number of protons.
Did you know?
Carbon-14 is an isotope used for dating objects and living materials in archeology.
What are the different types of isotopes?
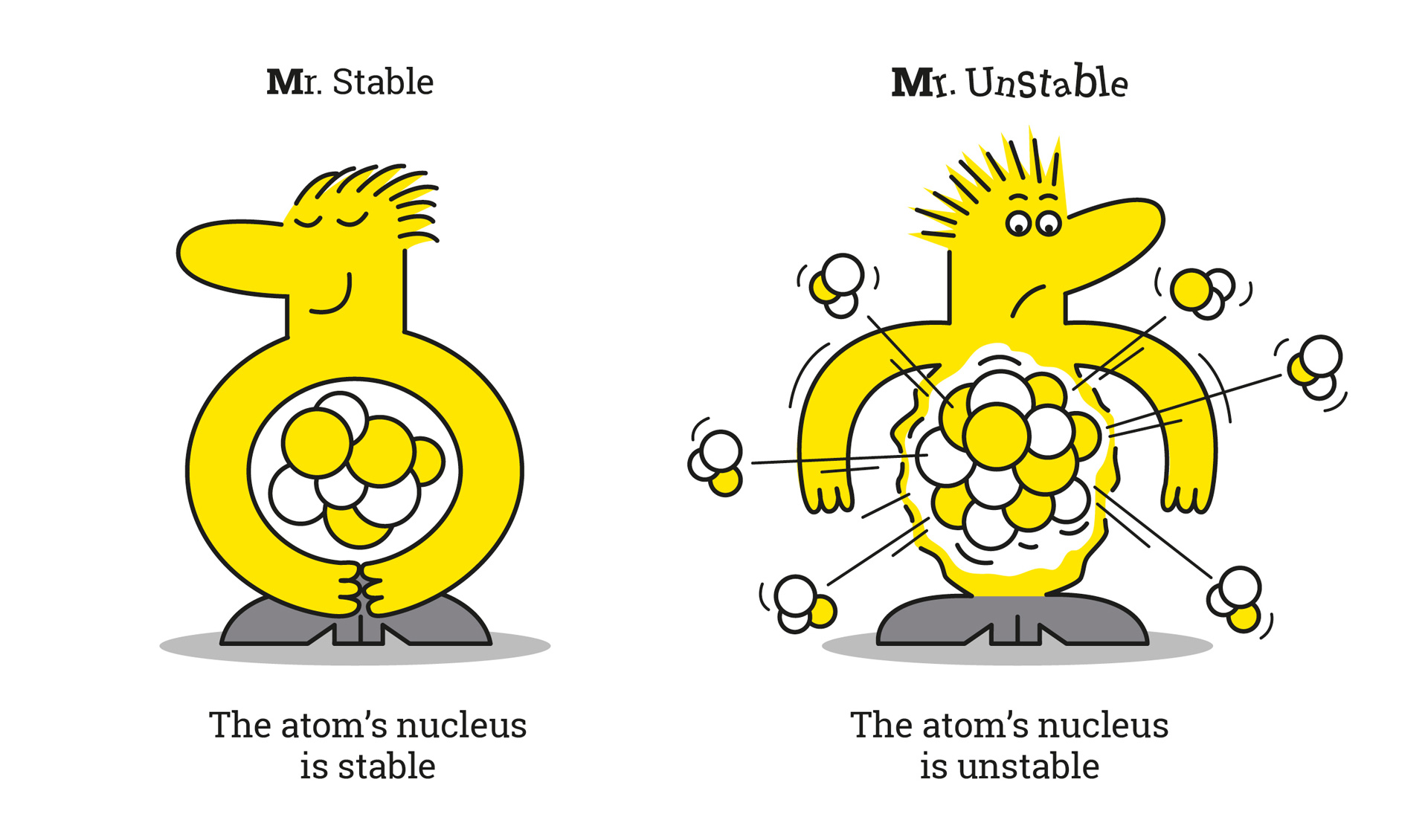
In science, isotopes are divided into two categories: so-called unstable or radioactive isotopes, and stable isotopes. These latter do not change and do not emit radioactive radiation.
However, some isotopes, such as Xenon, are known to be stable, while Xenon-124 and Xenon-136 degrade in several trillion years. The latter are thus considered stable on the scale of a human life.
Unstable isotopes contain surplus energy which they give off in the form of radiation. We speak of radioactivity.
Stable isotopes
Stable isotopes are defined by an association between protons and neutrons. They do not produce any radioactivity. It is the number of neutrons in the nucleus of the atom that guarantees this stability.Let's go back to the case of carbon-12, which is stable. It has 6 protons and 6 neutrons. As for carbon-14, it has 6 neutrons and 8 neutrons, which creates its instability.
Some examples of stable isotopes: isotopes of oxygen, hydrogen, or even nitrogen. Hydrogen has three isotopes: H, 2H and 3H. They each have one proton and a different number of neutrons.
Stable isotopes are mainly used in science, for example, for the study of the chemical composition of minerals. The use of stable isotopes helps scientists in the study of the origin of stones, or even metals. This is the case for carbon-12.
Stable isotopes are very promising in a range of applications: medicine, industry, basic research and quantum computing... They are at the heart of technological innovation.
-
Find out more about our activities in the Stable Isotopes LaboratoryLearn more
Unstable isotopes
Uranium isotopes: 234, 235 and 238, are among the most abundant and stable naturally-occurring isotopes.
Uranium isotopes: 232 and 236, for example, are produced in fission reactors. Along with uranium 233, they are among the artificially produced isotopes.
It is precisely this instability of the isotopes that is required for nuclear fuel. In particular, it contributes to generating nuclear fission reactions. Unstable isotopes of uranium are thus used as fuel for nuclear power plants.
Did you know?
The baseball team in the animated series The Simpsons is called “The Isotopes”. A nod to their sponsor, the Springfield nuclear power plant.
The birth of Mendeleev's Periodic Table
There are several families of isotopes, or chemical elements. In the 19th century, to classify them, the chemist Dmitry Mendeleev grouped them in the classification table of chemical elements. The latter are arranged by families of elements and by increasing atomic mass.At the start, only around 30 chemical elements were listed, against more than 80 by the end of the 19th century.
The table now has 118 elements, including about 325 natural isotopes and 1200 artificially produced isotopes.
The purpose of this table is to make it easier to identify the different chemical compounds by type of atoms.
-
The periodic table of the elementsFind out more
What are isotopes used for?
Unstable isotopes, also called radioactive or radioisotopes, have enabled major advances in sectors of industry, archeology with carbon-14 dating, but also in cancer treatments.
Isotopes and energy production

Nuclear energy, as we know it, was made possible by human intervention. Two industrial processes have been implemented: fusion and nuclear fission.
In the case of fusion, it is a matter of combining two nuclei of atoms
to form a larger atom and thus produce more energy.
In nuclear fission, the process involves the disintegration or splitting of a nucleus into smaller atomic nuclei. In fission, the Pu-239 isotope of plutonium is used, as well
as the U-235 isotope of uranium. When the nuclei of isotopes are split, they generate large quantities of energy.
Did you know?
Stable isotopes such as zinc, depleted in zinc-64, help reduce corrosion of nuclear reactor cooling equipment, while limiting the production of radioactive waste.
Unstable isotopes and cancer treatment

In the development of cancer treatments, the use of isotopes plays a major role, particularly in oncology.
An example of an innovative treatment is the use of a radioactive atom, lead-212. This is associated with biological
molecules, such as antibodies. As it disintegrates, the lead-212 isotope emits alpha radiation leading to the elimination of cancer cells and limiting damage to healthy cells.
This is a unique process called Targeted Alpha Therapy, developed in particular by Orano Med, the medical subsidiary of Orano, which launched
its first clinical trial in 2012.
Radioisotopes for medical imaging and medical treatments
 Radioisotopes are now an integral part of many procedures in medical diagnostics.
We find their use in the early detection of tumors, or even scintigraphy.
Radioisotopes are now an integral part of many procedures in medical diagnostics.
We find their use in the early detection of tumors, or even scintigraphy. In radioisotope scintigraphy, radioactive isotopes are used in the production of images. For example, using gamma rays equivalent to X-rays, their radiation promotes the taking of images of the targeted area during an examination.
Many medical radioisotopes currently used by nearly 35 million patients are, or can be, produced from irradiated stable isotopes.
80% of nuclear medicine procedures, such as lung scintigraphy, use radiopharmaceuticals, which improve diagnosis.
Unstable isotopes and archeology
 In addition to the dating of works of art with the use of the carbon-14 isotope,
isotopic analysis allows the identification of counterfeits, in particular by studying the dating of the work.
In addition to the dating of works of art with the use of the carbon-14 isotope,
isotopic analysis allows the identification of counterfeits, in particular by studying the dating of the work.
Isotopes are also used to preserve works by applying gamma radiation. Its aim is to slow down the deterioration of the work.
The use of isotopes in quantum computing
 The use of silicon-28 promises great advances in the industrialization of quantum chips with thousands or even millions of "qubits". To do this,
the researchers need silicon enriched in the isotope 28. Natural silicon is composed of 92% of the 28 isotope. After transformation, this will be increased up to 99.9%.
The use of silicon-28 promises great advances in the industrialization of quantum chips with thousands or even millions of "qubits". To do this,
the researchers need silicon enriched in the isotope 28. Natural silicon is composed of 92% of the 28 isotope. After transformation, this will be increased up to 99.9%.
Stable isotopes and fundamental research
 Stable isotopes serve a large number of sectors of the future such as quantum computing and fundamental experiments to improve the understanding of matter. For example, the isotope 136 of xenon (8.9% in its natural state) makes it possible to carry out research on matter.
Stable isotopes serve a large number of sectors of the future such as quantum computing and fundamental experiments to improve the understanding of matter. For example, the isotope 136 of xenon (8.9% in its natural state) makes it possible to carry out research on matter.
The nuclear sector's strategic isotopes
At the heart of the nuclear industry, a number of key isotopes are playing important roles in energy production, scientific research, and the development of innovative new solutions and applications.
At Orano, these isotopes, considered “strategic,”
are central to many of the group's activities, from the fuel cycle through research and nuclear medicine to the recovery and recycling of materials.
👉 Find out more in the fact sheets below!